Robustness traditionally has not been considered as a validation parameter in the strictest sense because usually it is investigated during method development once the method is at least partially optimized.

Meaning of robustness in method of validation.
Robustness is not only an indicator of good practice in method development but also a regulatory requirement.
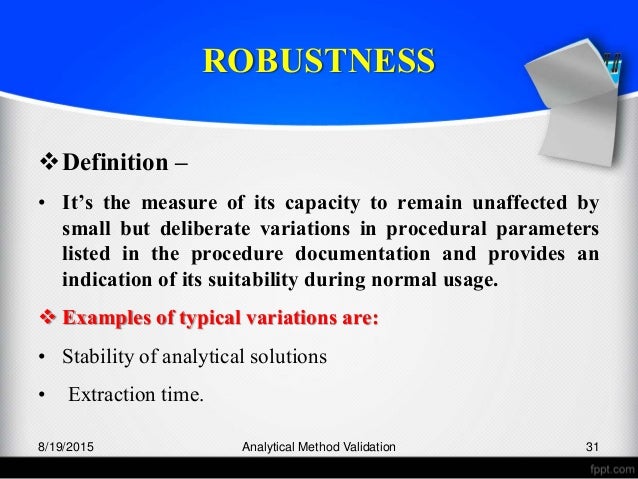
The purpose of a robustness study is to find out as much as possible about potential issues with a new analytical method and thus how it will perform in routine use.
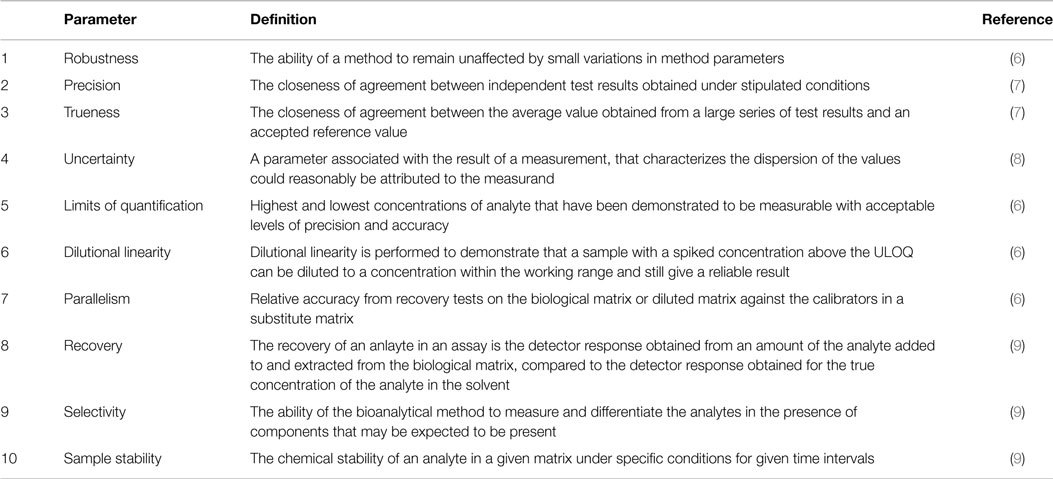
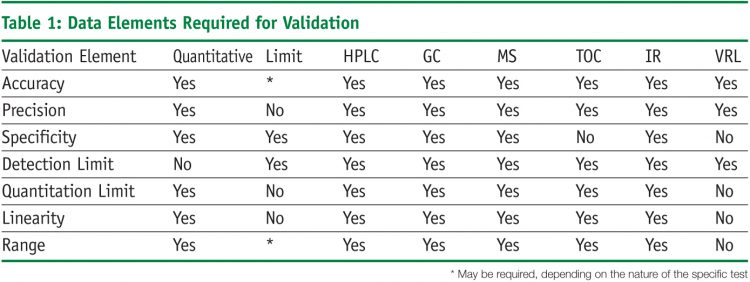
Ruggednessshould be used as a parameter evaluating constancy of the results when external factors such as analyst laboratory instrument reagents and days are varied robustnessshould be used as a parameter characterizing the stability of the method with respect to variations of the internal factors parameters of the method.
Robustness can encompass many areas of computer science such as robust programming robust machine learning and robust security networkformal techniques such as fuzz testing are essential to showing robustness since this type of testing involves invalid.
In this article we will address the same question through the parameter called robustness which can be evaluated during method validation if not yet done earlier eg.
Robustness data obtained during a methods development can be submitted in support of the validation of a method.
Robustness is the evaluation of an analytical method wherein the results obtained are found to be reliable even when performed in a slightly varied condition.
In computer science robustness is the ability of a computer system to cope with errors during execution and cope with erroneous input.
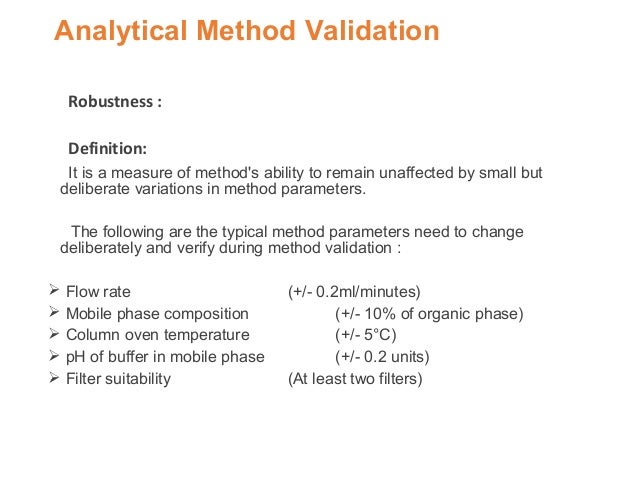
Usually we deliberately make changes in the method parameters to see if the method can still generate valid data.
It is the first stage of a robustness test to decide on which parameters should be tested and by how much to vary them.
In the usp the robustness of an analytical procedure is defined as a measure of its capacity to remain unaffected by small but deliberate variations in method parameters and provides an indication of its reliability in normal usage.

Https Www Metrology Journal Org Articles Ijmqe Pdf 2017 01 Ijmqe160046 Pdf

Validation Of Analytical Methods And Procedures

Pharmaceutical Method Development And Validation
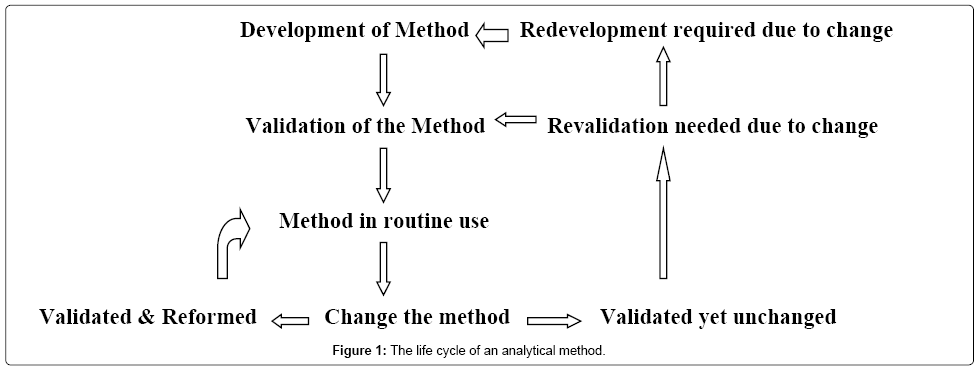
Analytical Method Development And Validation A Concise Review

Method Validation Approaches For Pharmaceutical Assessments

Test Method Validation The Starting Point Learnaboutgmp

Https Iqconsortium Org Images Publications Presentations Gmps Workshop Analytical Method Validation Regulatory Perspective Linda Ng Fda Pdf

Common Practices For Analytical Methods Transfer Pharmaceutical

Spe Method Validation Terms Precision And Accuracy Science

Analytical Method Validation For Biopharmaceuticals Intechopen
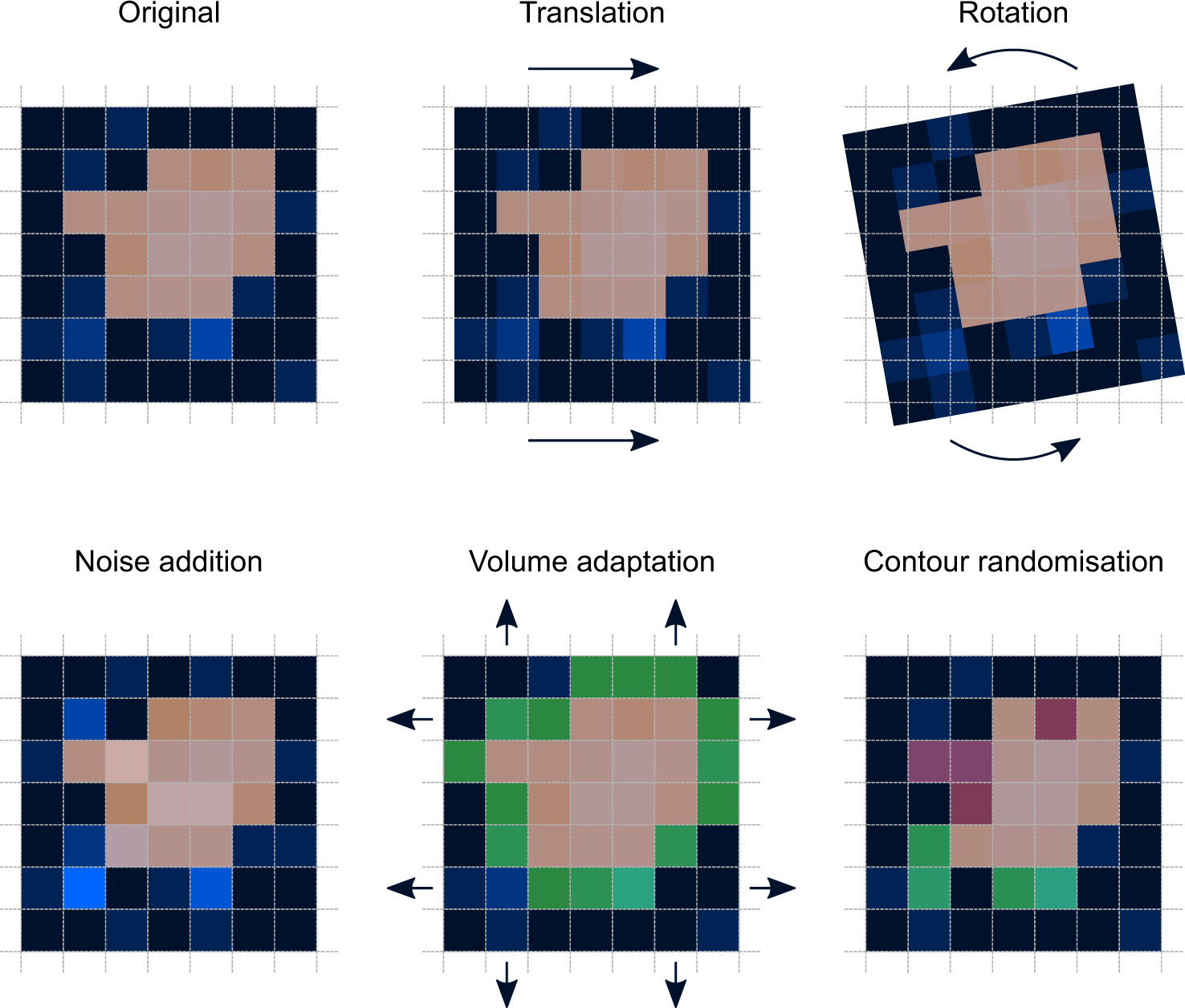
Assessing Robustness Of Radiomic Features By Image Perturbation

Validation Of Analytical Methods Based On Chromatographic

Http Grasasyaceites Revistas Csic Es Index Php Grasasyaceites Article Download 295 297

Lecture 10 Analytical Method Development And Validation In Hplc

What Is A Robust Method Chromatography Today

Https Arxiv Org Pdf 2006 01617

Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrk7hwhu Bj2l4b Sr0uotel5x Hlje1uva2zsb1krh35nbtze5 Usqp Cau

Http Www Demarcheiso17025 Com Document Validation 20of 20analytical 20methods 20based 20on 20chromatographic 20 20an 20overview Pdf

Establishing Acceptance Criteria For Analytical Methods Biopharm
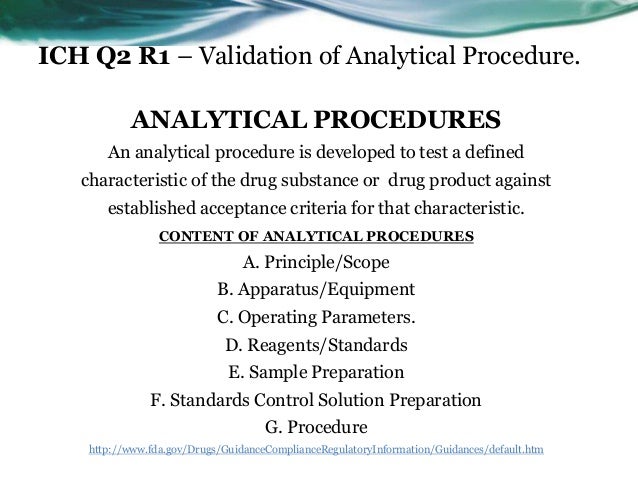
Ich Q2 Analytical Method Validation

Lecture 10 Analytical Method Development And Validation In Hplc
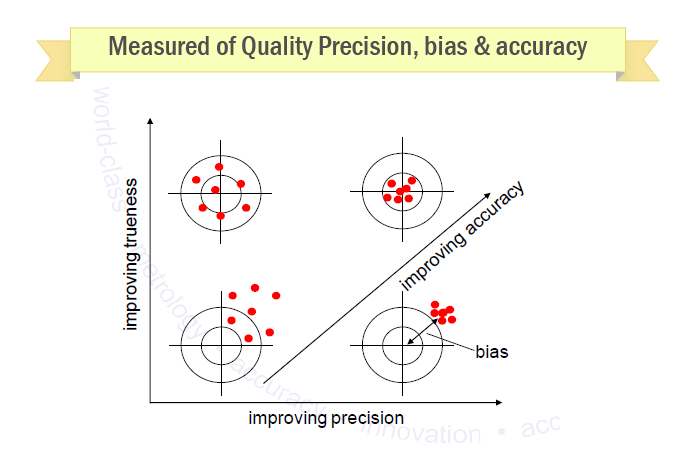
Method Validation What Is Method Validation How To Validate

Analytical Method Validation As Per Ich Vs Usp

Validation Of Analytical Methods And Procedures

Diagnostic Test Evaluation Methodology A Systematic Review Of

Validation Of Analytical Methods Based On Chromatographic

A Practical Guide To Analytical Method Validation Including

What Is Robustness

Establishing Acceptance Criteria For Analytical Methods

Analytical Method Validation

Pdf Validation Of Analytical Procedures Methodology Ich Q2b
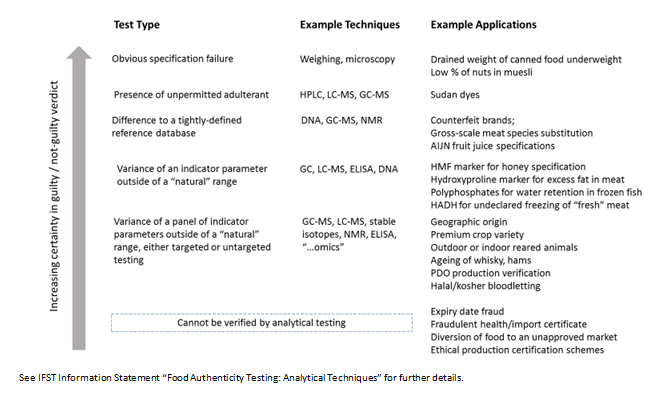
Food Authenticity Testing Part 1 The Role Of Analysis Ifst

Https Www Waters Com Webassets Cms Library Docs 720001826en Pdf

Validation Of Analytical Methods And Procedures

The Difference Between Verification And Validation Serendipity

Evaluating Optimal Therapy Robustness By Virtual Expansion Of A

Https Www Metrology Journal Org Articles Ijmqe Pdf 2017 01 Ijmqe160046 Pdf
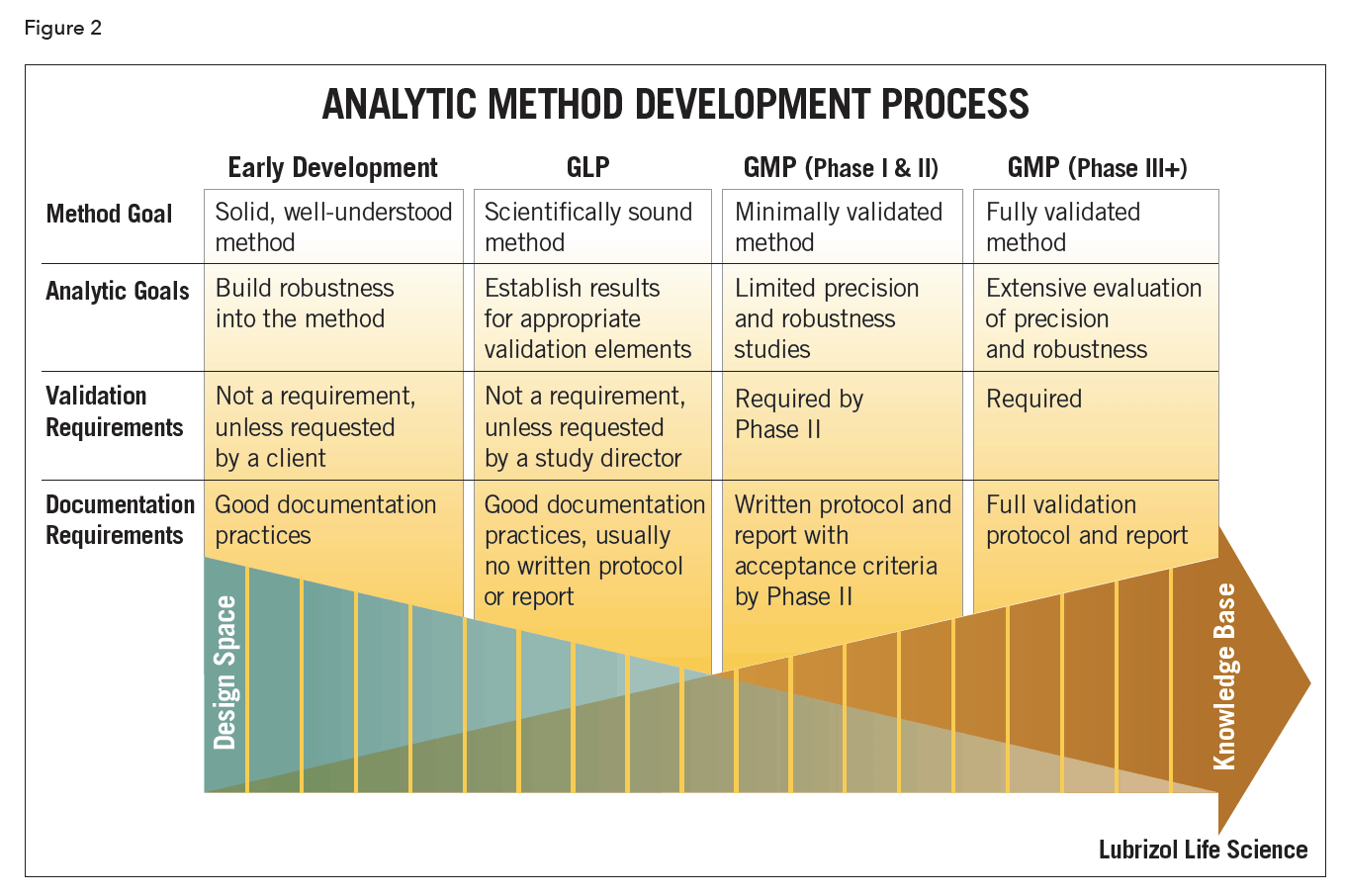
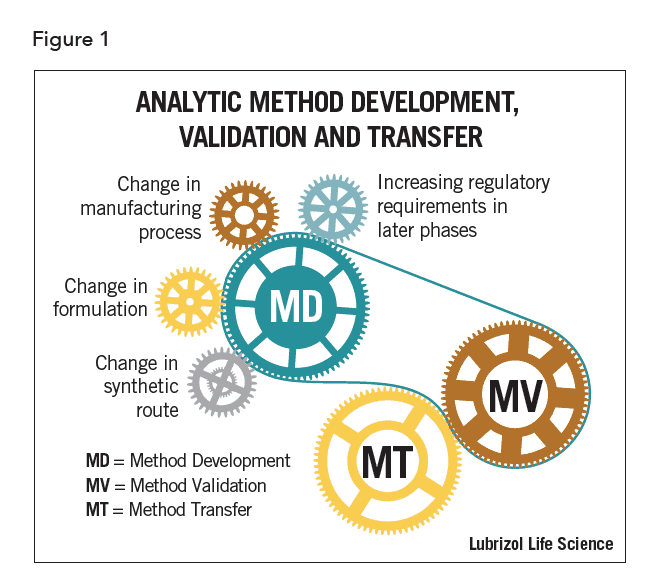
Analytical Method Development And Validation Lls Health Cdmo
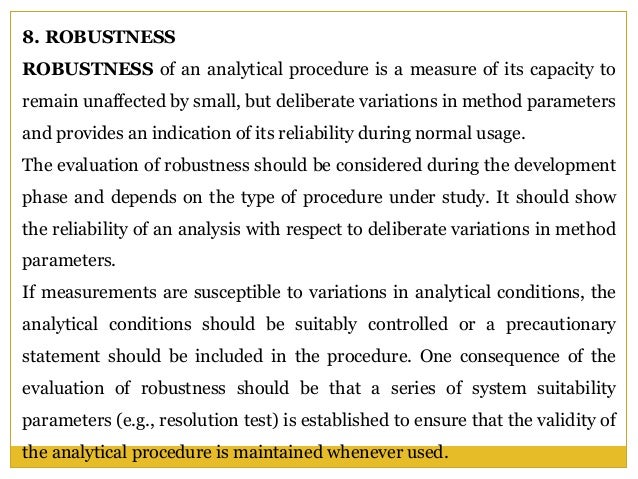
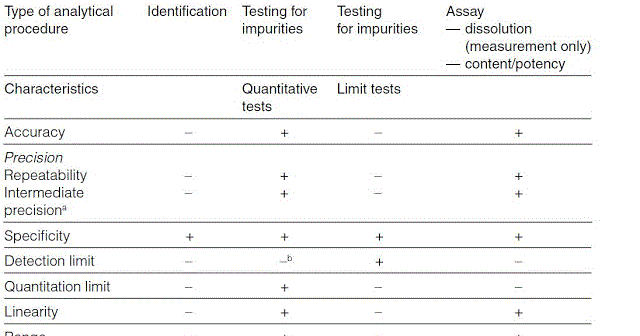
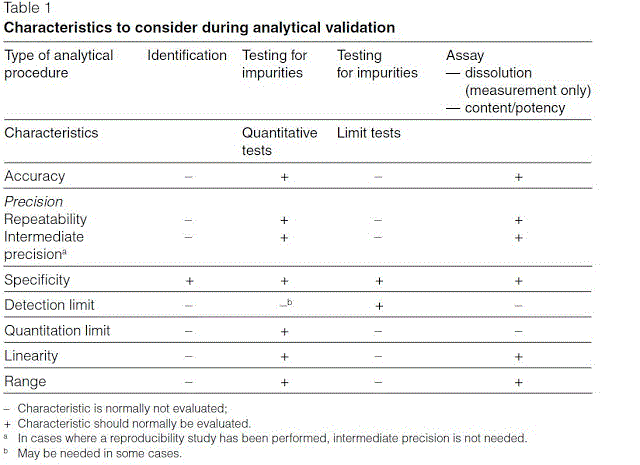
Validation Parameters
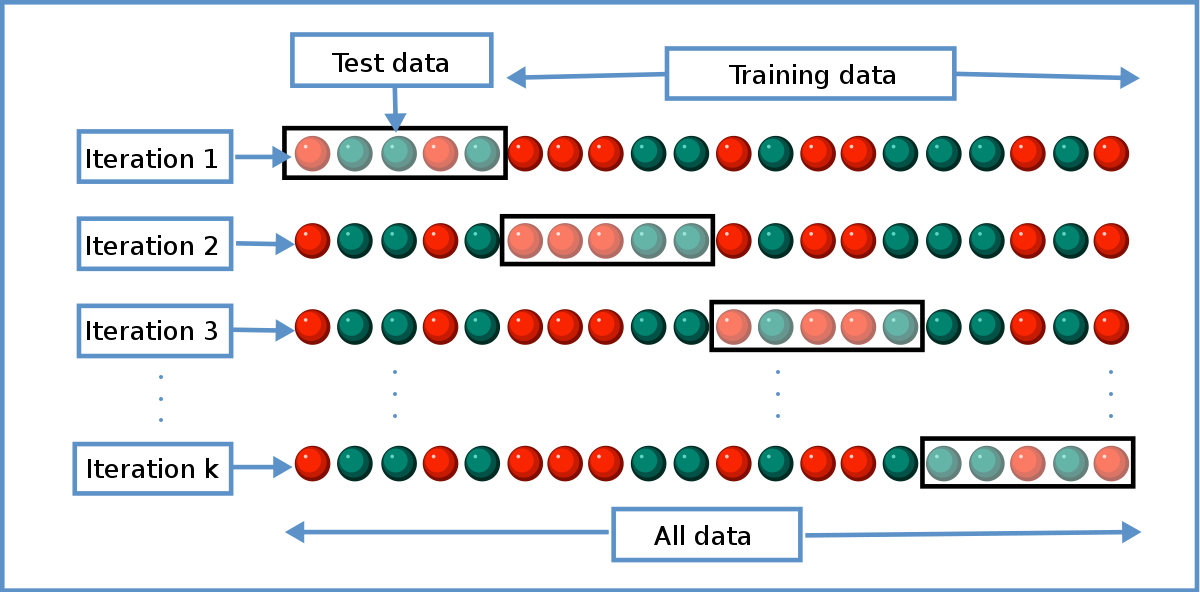
Cross Validation Statistics Wikipedia
Analytical Method Validation Pharmaceutical Guidelines
Analytical Method Validation Pharmaceutical Guidelines

A Systematic Method Development Strategy For Water Determinations

Robust Sage Research Methods

Https Www Epa Ie Pubs Conferencesandevents Aq Analytical Pdf

Https Www Waters Com Webassets Cms Library Docs 720001826en Pdf
Related Substances Method Validation Ppt Slide

Robustness And Ruggedness Introduction Youtube

Robust Statistics Wikipedia
Analytical Method Development And Validation Lls Health Cdmo

Method Validation And Robustness Lcgc

Https Onlinelibrary Wiley Com Doi Pdf 10 1002 Qaj 473
Making Predictive Models Robust Holdout Vs Cross Validation

Pdf Hplc Method Validation For Pharmaceuticals A Review

Validation Of Analytical Methods Intechopen

Is It Really Necessary To Validate An Analytical Method Or Not

Robustness Diagrams An Agile Introduction

Genomic Organization Underlying Deletional Robustness In Bacterial

Analytical Method Validation By Manoj Ingale Best Ppts

Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsbek6ewivlfbup22km4iikkuu3pytvd5y Rr7cvg1tggnw3gz6 Usqp Cau
Analytical Method Development And Validation Lls Health Cdmo

3 3 Estimating The Linear Range Mooc Validation Of Liquid
Robust Regression Wikipedia
Pdf Analytical Method Validation

Pharmaceutical Method Development And Validation

Https Sisu Ut Ee Sites Default Files Lcms Method Validation Files Lcms Pdf 161017 Pdf

Bridging Analytical Methods For Release And Stability Testing

Method Validation And Robustness Lcgc

Https Cdn Ymaws Com Www Casss Org Resource Resmgr Cmc No Am Jan Spkr Slds 2018 Cmcj Khrenovalexey Pdf
Analytical Method Validation By Manoj Ingale Best Ppts

Analytical Quality By Design Aqbd In Pharmaceutical Development
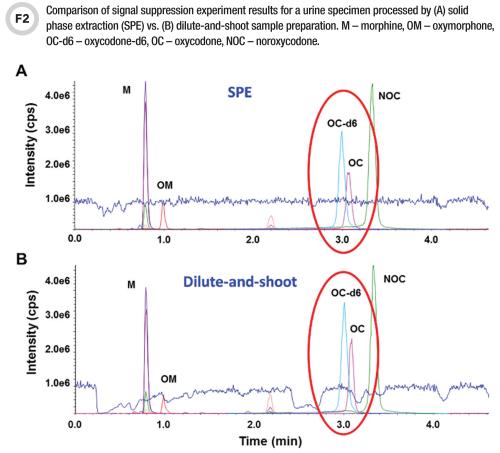
Interference Testing And Mitigation In Lc Ms Ms Assays Aacc Org

Method Validation And Robustness Lcgc

Https Www Waters Com Webassets Cms Library Docs 720001826en Pdf

Development And Validation Of An Hplc Method For Determination Of
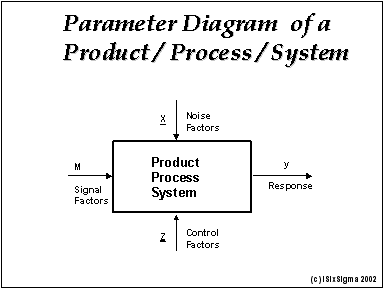
Introduction To Robust Design Taguchi Method

Top 250 Method Validation Interview Questions And Answers 07 July
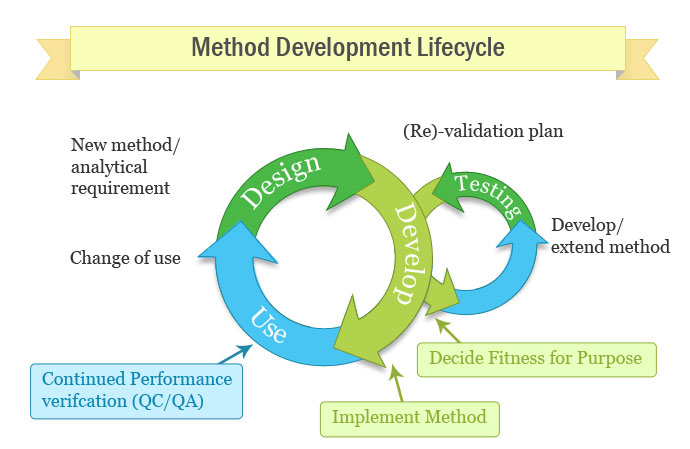
Method Validation What Is Method Validation How To Validate

Analytical Quality By Design Aqbd In Pharmaceutical Development

Validation Of Analytical Methods And Procedures

Https Www Fda Gov Media 83812 Download
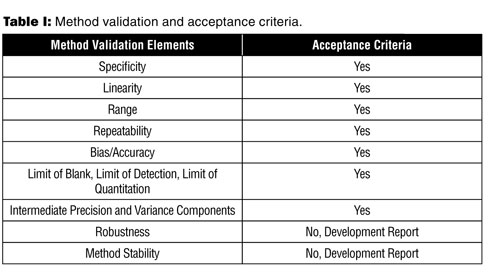
Establishing Acceptance Criteria For Analytical Methods

Guide To Method Validation Of Test Procedures Labcompare

Guidance For Robustness Ruggedness Tests In Method Validation

Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcq5najpkbiwbsbdmfziec Ightz9vowh9wab8pda2q Smujsy85 Usqp Cau

Robustness And Ruggedness Introduction Youtube

Pdf Robustness Evaluation In Analytical Methods Optimized Using
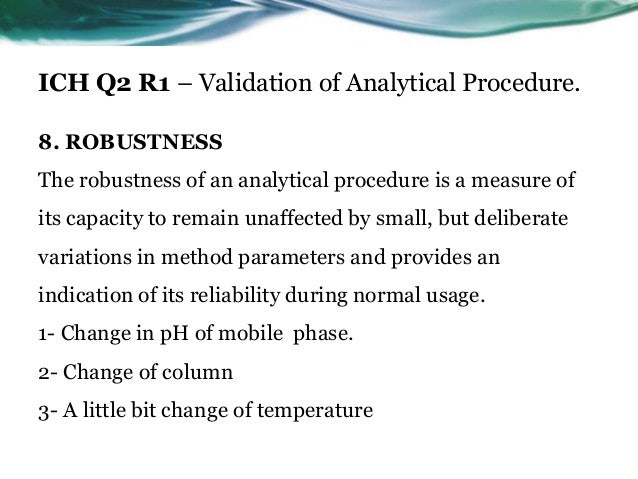
Ich Q2 Analytical Method Validation
Robustness Diagram

Http Iosrphr Org Papers V5i10 B051007019 Pdf

Https Pdf4pro Com File 1c717 Wp Content Uploads 2013 12 Usp36 1225 Pdf Pdf

Machine Learning Algorithm Validation With A Limited Sample Size

7 Quality Of Analytical Procedures

Is It Really Necessary To Validate An Analytical Method Or Not

Guidelines For Analytical Method Validation How To Avoid